Physicists calculate stability of trifluorides of rare earth elements under pressure
Laboratory of Computer Design of New Materials and Machine Learning of the Institute of Physics has carried out comprehensive studies of typical trifluorides of rare earth elements – gadolinium (GdF₃), terbium (TbF₃), dysprosium (DyF₃), holmium (HoF₃), and erbium (ErF₃) under high pressure up to 30 GPa. The research was a part of the Priority 2030 strategic academic leadership program.
A trifluoride is a chemical compound in which three fluorine (F) atoms or ions are bonded to one atom or ion of one element. It is formed by many metals, in particular, a group of rare earth elements. The unique properties of the compounds determine their value for a wide range of applications in biomedicine, laser technology, catalysis, hydrogen storage and other high-tech fields.
The role of trifluorides is significantly enhanced if the compounds have the form of nanoparticles, says Lead Research Associate Irina Gumarova. According to her, a number of previously published studies have confirmed their potential use in biomedical applications such as computed tomography and magnetic resonance imaging.
“In-depth study of trifluorides under high pressure allows us to understand how their structure is related to physical properties, which is important for optimizing the use of these compounds and developing materials with specified characteristics,” adds the physicist. “Of particular interest are the phase transitions under pressure and the stability of the materials, as these parameters directly affect the possibility of their application in high-tech.”
KFU scientists took part in the study, including Senior Research Associate Regina Burganova, Irina Gumarova, Associate Professor Oleg Nedopekin, and co-authors Zafari Umar, researcher at the Center for Innovative Development of Science and New Technologies of the National Academy of Sciences of Tajikistan, and Ilya Chepkasov, Senior Research Associate at the Skolkovo Institute of Science and Technology.
The aim was to obtain the characteristics of selected trifluoride systems by quantum mechanical calculations. The main task was to create a theoretical basis for experiments and further calculations related to trifluorides both in crystalline form and in the form of nanocrystals.
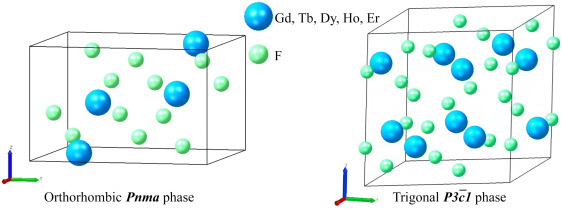
Thus, for the first time, the scientists selected an optimal set of modeling parameters universal for the entire family of REE trifluorides; the mechanical stability of compounds, elastic characteristics and evolution of lattice parameters under pressure changes were investigated.
“Based on a comparative analysis of elastic constants, it was found that gadolinium, terbium, dysprosium, holmium, and erbium trifluorides in the orthorhombic phase show greater stability to unidirectional compression than to shear deformation. The same behavior is observed in the trigonal phase,” Burganova notes.
Calculations based on the proposed parameters and modeling methods carried out at KFU confirm the presence of phase transitions under pressure, the values of transition points of which agree with the available experimental data. The analysis of electronic, magnetic and optical properties of all five trifluorides proved the uniqueness of these compounds.

